Authors: Suzana Žunec, Donna Vadlja, Alma Ramić, Antonio Zandona, Nikola Maraković, Iva Brekalo, Ines Primožič, Maja Katalinić
Abstract
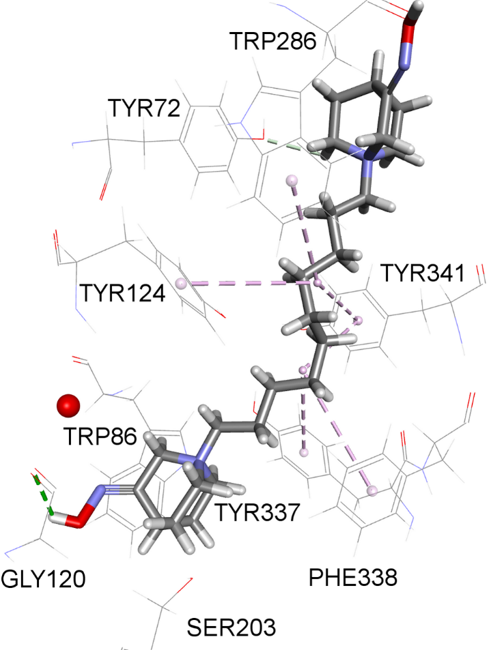
The cholinergic system, relying on the neurotransmitter acetylcholine (ACh), plays a significant role in muscle contraction, cognition, and autonomic nervous system regulation. The enzymes acetylcholinesterase, AChE, and butyrylcholinesterase, BChE, responsible for hydrolyzing ACh, can fine-tune the cholinergic system’s activity and are, therefore, excellent pharmacological targets to address a range of medical conditions. We designed, synthesized, and profiled 14 N-alkyl quaternary quinuclidines as inhibitors of human AChE and BChE and analyzed their impact on cell viability to assess their safety in the context of application as potential therapeutics. Our results showed that all of the 14 tested quinuclidines inhibited both AChE and BChE in the micromolar range (Ki = 0.26 − 156.2 μM). The highest inhibition potency was observed for two bisquaternary derivatives, 7 (1,1′-(decano)bis(3-hydroxyquinuclidinium bromide)) and 14 (1,1′-(decano)bis(3-hydroxyiminoquinuclidinium bromide)). The cytotoxic effect within 7–200 μM was observed only for monoquaternary quinuclidine derivatives, especially those with the C12–C16 alkyl chain. Further analysis revealed a time-independent mechanism of action, significant LDH release, and a decrease in the cells’ mitochondrial membrane potential. Taking all results into consideration, we can confirm that a quinuclidine core presents a good scaffold for cholinesterase binding and that two bisquaternary quinuclidine derivatives could be considered as candidates worth further investigations as drugs acting in the cholinergic system. On the other hand, specific cell-related effects probably triggered by the free long alkyl chain in monoquaternary quinuclidine derivatives should not be neglected in future N-alkyl quaternary quinuclidine derivative structure refinements. Such an effect and their potential to interact with other specific targets, as indicated by a pharmacophore model, open up a new perspective for future investigations of these compounds’ scaffold in the treatment of specific conditions and diseases other than cholinergic system-linked disorders.
The article is available at the following link.

Comments